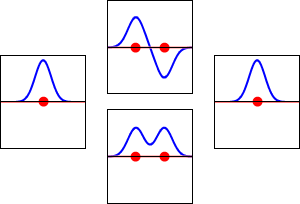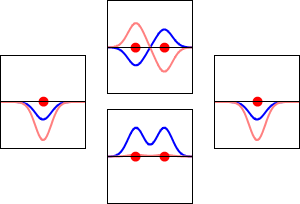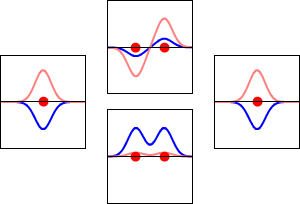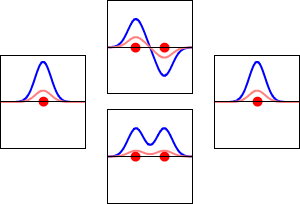
8 - Drawing Molecular Orbital Diagrams
Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Although more complex, these diagrams reveal a more realistic case for bonding, allowing electrons to travel about a molecule, rather than in between one.
7 - Advanced Theories of Covalent Bonding
Valence bond theory, the theory that explains bonding through the use of lone pairs and electrons between nuclei, is convenient for explaining basic bonding. However, when we consider the wave-like behavior of electrons, it falls short in explaining the reality behind covalent bonding. This lesson moves us forward with a new theory - molecular orbital theory.
6 - Sigma and Pi Covalent Bonds
Hybridization, beyond giving us a reason for covalent bonding, also helps us understand that there are two different types of covalent bonds - the sigma and the pi bond.
5 - Bonding Orbitals and Hybridization
Hybridization, or the overlapping of orbitals to form new, hybrid orbitals, is a valuable missing piece to our understanding of covalent bonding.
4 - VSEPR Theory and Molecular Geometry
Did you know that molecules take different shapes based on the bonds that are formed? The VSEPR Theory helps us organize molecules into particular shapes according to how their electrons are distributed throughout the molecule.
3 - Classification of Carbon Molecules
Carbon forms the skeleton of all life on Earth due to its flexible nature. This part discusses the carbon’s flexibility through both its degree and priority and finishes our discussion on molecular naming.
2 - Functional Groups and Structures
Organic molecules are named using their functional groups, groups of bonded atoms that have distinct chemical properties. Here, we cover a few of those functional groups.
1 - Chemical Naming System
Just as atoms have their own rules for which atoms bond together and how, molecules also adhere to rules. But, before we broach those, where do the names of these molecules come from? Is it a complex naming system and is any information revealed within a name?
4 - Properties of Metals, Non-metals and Metalloids
The dances of chemistry can really be spectacular and unique. The best part is, the periodic table gives you a hint into the kinds of bonds that might happen.
3 - Electronegativity of an Atom
We define a bond as something that ties things together. It's easy to tell who our bonds lie with, but, atoms have bonds as well. These are the dances of chemistry.