3 - Classification of Carbon Molecules
Abstract (TL;DR):
This lesson discusses the way we number carbon atoms in molecules by understanding carbon priority and carbon degree.
Carbon priority is determined by how many carbon atoms are bonded to the carbon atom of choice with first degree carbons being bonded to one other carbon, second degree carbons being bonded to two and third degree carbons being bonded to three. According to carbon priority, the name of the molecule can change.
Sometimes, however, certain molecules can have different configurations, which changes a carbon’s priority, but, generally, is the same molecule by name. To properly label differences, we use carbon degree to properly number our carbons, giving us a detailed name.
——————————-
Coal: caused by a particular configuration of carbon and other molecules.
We’re almost done with organic compounds, however, there are a few loose ends to tie up.
You might have thought, seeing those molecules in the last part, that those were the only configurations that they could be in. But that’s not the case - a functional group is not limited to the end of an alkyl chain. What if the functional group connected to the middle of a chain?
It turns out that the functional group’s position influences the molecule’s name. To understand how, we need to talk about how carbon atoms are classified according to two principles: priority and degree.
Carbon Classification
For classification of carbon-based molecules, we’ll use alcohols (alkyl + hydroxyl functional group (-OH)), since they’re simple.
Degree
According to how many carbons atoms are bonded to the carbon attached to the hydroxyl group, the resulting molecule can be a primary, secondary or tertiary alcohol.
Primary (1°) alcohols only have one carbon attached to the carbon connected to the hydroxyl group. Ethanol is the simplest primary alcohol.
Second (2°) alcohols have two carbons attached to the carbon connected to the hydroxyl group. Propanol is the simplest secondary alcohol.
Tertiary (3°) alcohols have three other carbons attached to the carbon connected to the hydroxyl group. Butanol is the simplest tertiary alcohol.
Priority
You might notice that, with propanol or butanol, the -OH group could be put on multiple carbons. That simple change creates an entirely different molecule. But how do you tell all the configurations of propanol apart when it can be a primary or secondary alcohol? How about butanol, which can be primary, secondary or tertiary?
First, you must realize that there is no “true” structure to many of these molecules. Rather, it’s the carbon priority that determines what any given structure is called.
Carbon priority puts a number to the carbons on the molecule’s parent chain. According to the number that the substituent is attached to, we can provide a specific label to a specific degree of molecule.
Let’s take a look at a 2° butanol molecule and figure out how to number carbons.
To figure out carbon priority, we first need to figure out the parent carbon chain, which, simply, is the longest chain of carbon atoms. The parent chain is where we number our carbons. In the above molecule, we can see that we have a chain of four carbons.
But which end of the chain do you start from? When giving your molecule a name, it turns out that the answer to this question matters above many other things. To choose the proper starting point, we look for the carbon closest to the first substituent. So, in this butanol molecule, the -OH functional group is closest to the carbon on the bottom, meaning that carbon is carbon #1. Then, we simply number our carbons from that carbon along the rest of the chain.
In this butanol, the functional group is on the second carbon. Therefore, we simply name this molecule 2-butanol, since the hydroxyl group is attached to the second carbon in the four carbon molecule.
Complex Molecule Names
There are a few other situations that you might run into when seeing a molecule.
Multiple Substituents on a Parent Chain
The Same Substituent
Here we have a molecule with two hydroxyls. When we have only one in our molecule, it has the suffix “-ol”, like the alcohols we have covered so far. But when we have two, the suffix changes to “-diol” (“di” coming from the Greek word dis meaning “two”).
| Prefix | |
|---|---|
| 1 | ~ |
| 2 | -di |
| 3 | -tri |
| 4 | -tetra |
| 5 | -penta |
| 6 | -hexa |
| 7 | -hepta |
| 8 | -octa |
In fact, all substituent suffixes and molecule prefixes have an additional prefix (-di, -tri, -tetra) if there are more than one of either. Two alkenes in a molecule, for example, would be a diene, three aminos would be a triamine and so on.
For molecule parts, we add this prefix in addition to the prefixes that connote the number of carbons in a molecule (meth-, eth-, prop-).
In any event, back to the molecule. The process is essentially the same as above. First, we identify and number the parent chain, according to the end nearest to the first substituent. Since the carbon on the right of the molecule is attached to one of the two hydroxyl groups, that would be our #1 carbon.
Since there are two functional groups here, the name also changes to reflect that. The hydroxyl functional groups are on the first and third carbon in our chain. So, the name of this molecule is 1,3-butanediol.
Different Substituents
This one is a little more difficult to dissect, but, again, we follow the same principles: (1) identify the parent chain, then (2) number the carbons in your molecule according to the carbon (or carbons) nearest to the first substituent.
Don’t be fooled by its appearance; this is not a chain of four carbons attached to two methyl groups and a hydroxyl group. The parent chain includes all carbons. In this molecule, one of the methyl carbons becomes the first carbon in the chain because it is the closest end carbon to the hydroxyl and the other methyl group.
Finally, since there are different substituents, the name you put first is determined alphabetically, ignoring the prefixes caused by multiple substituents, like those in the table above. With all of that said, this molecule’s name is 2-methyl-2-pentanol.
The Presence of Alkenes and Alkynes
What about the case where your parent chain has double bonds and triple bonds? There are more interesting rules for these molecules. But, before we get to those, let’s throw up a simple molecule.
This molecule contains two alkenes (double-bonds between carbons). We number the first carbon in the parent chain (and the only chain, in this case) by finding the carbon that gives the alkenes their lowest numbers. Therefore, the above molecule is a 1,4-hexadiene (don’t forget, two alkenes is a diene).
What if there was an alkyne (triple bonded) added to the mix?
| Infix | |
|---|---|
| 1 | -an |
| 2 | -en |
| 3 | -yn |
This is called 1,3-hexadien-5-yne. I know. You’re thinking, “that can’t be right.” But it is.
In the name, you might have noticed that the e on the “-ene” infix was left out. That was purposeful. When an alkane, alkene or alkyne suffix comes before a number, you drop the last “e” (check the table on the right for the appropriate infix in these situations).
You also might have noticed that we only included the alkyne’s suffix (-yne). Well, the first “hexa” already covers the six carbons; there’s no need to repeat ourselves with a cumbersome name like 1,3-hexadien-5-hexyne - only include the suffix. That goes for any functional group, by the way, not just alkynes.
In the molecule, there are two alkenes and one alkyne in a six-carbon molecule. Like with 1,4-hexadiene, we look for the carbon near the alkene (or alkyne) that gives the lowest number and start there. In cases where the alkene and alkyne both have the same number, like they would above, we give the alkene priority.
Substituents With the Same Length As a Parent Chain
We know that a parent chain is the longest chain of carbons. But what if there is a substituent that has the same amount of carbons as a parent chain?
What’s the parent chain and what’s the substituent? That’s the question, isn’t it. No matter where you look, the parent chain can begin from any end.
There are a four ways to break this tie, according to the IUPAC[1]. In order of highest to lowest priority, parent chains are chosen according to the chain that:
- Has the largest number of substituents attached to it.
- Has the lowest-numbered substituents (the normal condition).
- Supports alkyl substituents with the largest number of carbons in them.
- Has substituents that have a low number of branches themselves.
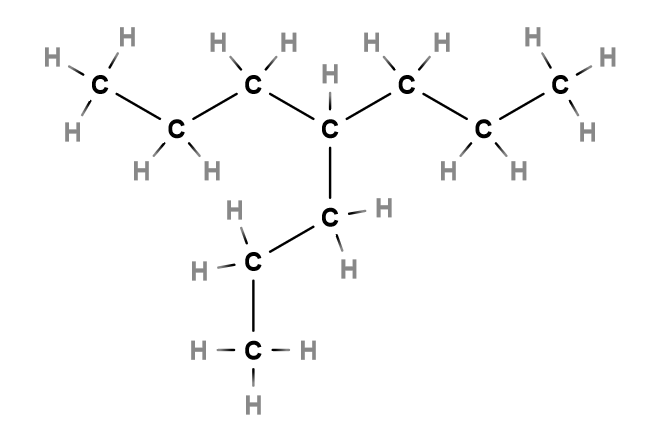
So, when we can’t figure out which carbon has the substituent on its lowest number, we decide by finding which carbon supports the alkyl substituent with the most carbons. Don’t get distracted by the strange molecular beast made of three arms. No matter which of the three ends you choose, there are seven carbons in the parent chain and a three-carbon alkyl substituent, better known as propyl, attached to the fourth carbon.
We can tell that part of this name is “4-propyl”, but what about the seven carbons we didn’t account for? Remember your general formulas for alkyl group molecules? If you count all the carbons in the molecule, you find that there are ten total. It also happens to contain 22 hydrogens. This fits the formula CnH2n+2, making it an alkane!
* Naming Priority for Hydrocarbons
When naming a molecule, if there are two or more of the hydrocarbon present, the priority is: (1) alkyl, (2) alkane, (3) alkene, (4) alkyne. Don’t forget about alphabetical order if there are two of any given type!
Therefore, the seven carbons that we didn’t account for can be included with its alkane name - heptane. The whole name of the molecule is 4-propylheptane; in this case, the alkyl name takes priority over the alkane name*.
Bringing It All Together
The Nomenclature of Organic Molecules, commonly called the “Blue Book”, holds all even more rules that I haven’t covered here. It would truly be a saga to go over each of them. The ones that I have covered, however, are the most common.
But, now that we have covered the way these molecules are named, it’s time to get into the meat of the matter - how these molecules behave and why.
Until then, please take your time in reviewing these last two sections; they’re definitely dense. Ask questions if you have them and, please, abide by the appropriate guidelines on how to handle the current COVID-19 pandemic. Stay safe.
Cited:
[1] Favre, H. A., Powell, W. H., & International Union of Pure and Applied. (2013). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (International Union of Pure and Applied Chemistry) (1st ed.). Retrieved from https://www.acdlabs.com/iupac/nomenclature/79/r79_36.htm
Read the CDC Guidelines on how to protect yourself during the current COVID-19 pandemic.
For more thought-provoking content, join our mailing list!
See Flux’s Plans for 2020 here!