2 - Functional Groups and Structures
Abstract (TL;DR)
There are a few rules for naming organic compounds that before we begin. We must understand functional groups to understand the names of organic compounds. Functional groups, groups of particular molecules bonded to a chain of carbons, all give molecules particular characteristics. In this article, we’ll go over just a few.
—
Vinyl (a substance created by one of the functional groups below)
Since organic compounds always contain carbon and hydrogen, their naming scheme is far more predictable. However, these molecules can have far longer and messier names. Because of this, many organic compounds have cleaner nicknames. For the organic compounds I name, I’ll also provide its nickname, if they have one.
Functional Groups
First, since carbon has both four valence electrons and needs four electrons to reach its next noble gas, it is a very flexible atom (which is why all life on Earth uses it as a backbone). Carbon very easily bonds with itself, but often also bonds with hydrogen, an atom that covalently bonds to be stable (like its nearest noble gas - helium). The stability of the hydrogen-carbon bond allows for long hydrocarbon chains to form. According to the number of carbons in these chains, we know which prefix to use for a molecule’s name.
| Number of Carbons | Molecule Prefix |
|---|---|
| 1 | meth- |
| 2 | eth- |
| 3 | prop- |
| 4 | but- |
| 5 | pent- |
| 6 | hex- |
| 7 | hept- |
| 8 | oct- |
| 9 | non- |
| 10 | dec- |
Not all of the bonds in a hydrocarbon chain have to be hydrogen, of course. In fact, at any part in this chain, a different atom or molecule can latch on. In this case, the hydrocarbon chain that other molecules latch onto is called the parent chain, while the molecules that latch on are called substituents. Substituents replace the hydrogens that normally connect to carbons in the hydrocarbon chain.
The flexibility in carbon bonding gives rise to the idea of functional groups, particular substituents that bond to hydrocarbon chains. The general formula of a molecule with a functional group is RG, where R is a molecule and G is the functional group.
Below, we’ll list some of the most common functional groups, how they affect the chemical’s name, their general chemical formulas and even some major types of molecules that these functional groups form.
Alkyls
The first and simplest functional group are alkyls. They are the example of the hydrocarbon described above with space for substituents. Alkyls make room by only bonding three hydrogens to a carbon instead of the full four, giving carbon the ability to bond to something else. We call those carbons unsaturated, while the carbons that have the full four are saturated. The name of any alkyl includes the suffix “-yl”.
The alkyl group has a general formula CnH2n+1-, where n represents some number. In other words, if there is 1 carbon, there is 3 hydrogens in the alkyl group (CH3-). The name of this molecule would be “methyl”. C2H5- is called “ethyl”. And so on.
An alkane is created in the case where a hydrogen attaches to an alkyl and forms the full hydrocarbon. All alkanes contain the suffix “-ane” in their names. The simplest alkane, therefore, would be CH4 carbon bonded to four hydrogens. This alkane is called “methane” - the familiar greenhouse gas and primary part of natural gas.
Alkenyls and Alkynyls
Up until this arc, we have only focused on single covalent bonds between two atoms. However, so long as an atom has enough valence electrons to share with another atom, it can form two or even three bonds. A few transition metals can even make four, stable bonds between two atoms!
Adding these extra bonds to an alkyl (and removing hydrogens from the bonded carbons), creates the alkenyl and alkynyl functional groups.
Fun-fact: the simplest alkenyl, called ethynyl (two carbons double-bonded), is also called vinyl. Adding a chlorine to this instead of hydrogen creates vinyl chloride, commonly known as vinyl.
Alkenyls have double bonds in their hydrocarbon chain and, like alkyls, have an unsaturated carbon. They have the general formula CnH2n-1-. Alkynyls have triple bonds and also have an unsaturated carbon. The alkynyl group has the general formula CnH2n-3-.
Also like alkyls, if you saturate the molecule with hydrogens, you create two classes of molecules - alkenes and alkynes.
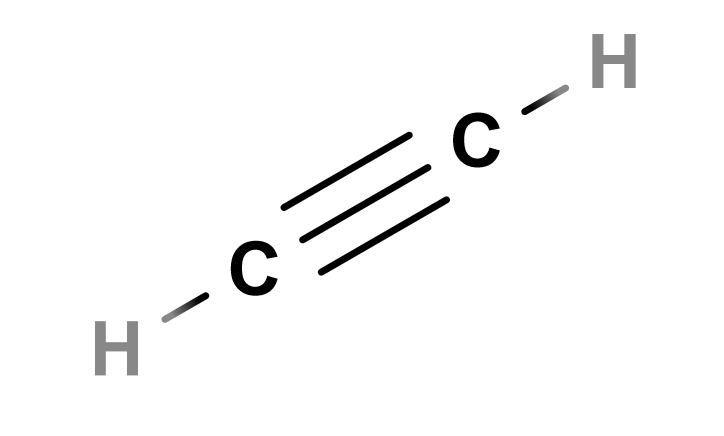
Lewis Structure of Ethyne
Alkenes have the general formula CnH2n and its molecules have the suffix "-ene". Alkynes have the formula CnH2n-2 and molecules including them have the suffix "-yne" in their name.
Ethyne, also known as acetylene, is a common, albeit unstable fuel source that’s popular for tasks like cutting and welding.
Hydroxyls
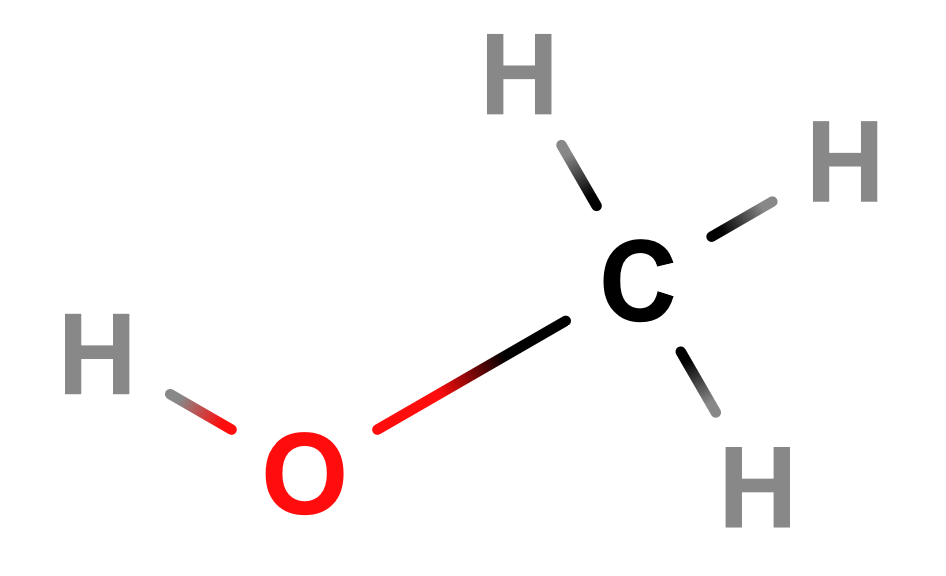
Lewis Structure of Methanol
Hydroxyls are a functional group that contain an -OH substituent. They combine with an alkyl to form the familiar class of molecule called alcohols (general formula: R-OH). The name of any alcohol includes the suffix “-ol”. For the prefix, an alcohol uses the name of the alkane that corresponds to the number of carbons it has.
With this information, we can name simple alcohols. If only one carbon is bonded to the hydroxyl group, the molecule is called “methanol”, the well-known fuel. If there are two carbons in the chain, then the molecule is called “ethanol”, best known as the alcohol you taste in beverages. Three carbons is propanol. And so on.
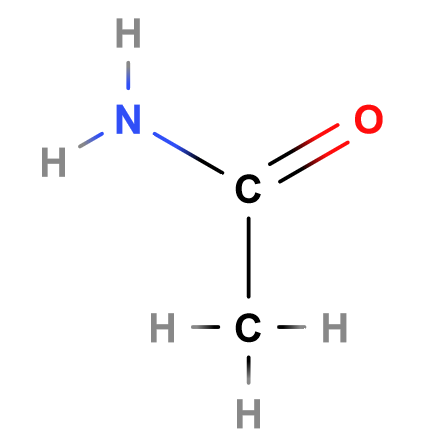
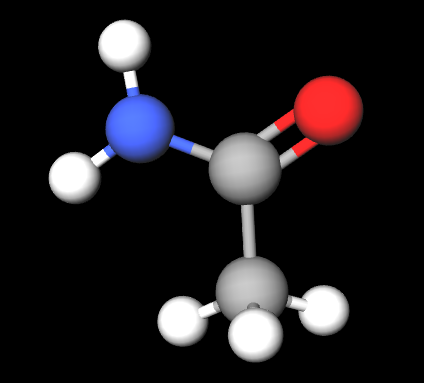
Aminos
Next - aminos. The amino group is made up of an -NH2 substituent. This group bonds with an alkyl parent chain to form amines (general formula: R3N, where each R is either an alkyl or a hydrogen). Note that, unlike oxygen, nitrogen bonds with three atoms to complete its valence shell (whether that be carbon or hydrogen).
The name of any amine contains the suffix “-amine” (easy to remember). Unlike alcohols, amines take the name of the entire alkyl base as the prefix.
The above, for example, is propylamine - the amino group is attached to a propyl alkyl. Amines are, perhaps, the most important functional group in an organism’s body. They are necessary for not only our cell’s growth and our metabolism, but also the structure of our DNA and proteins.
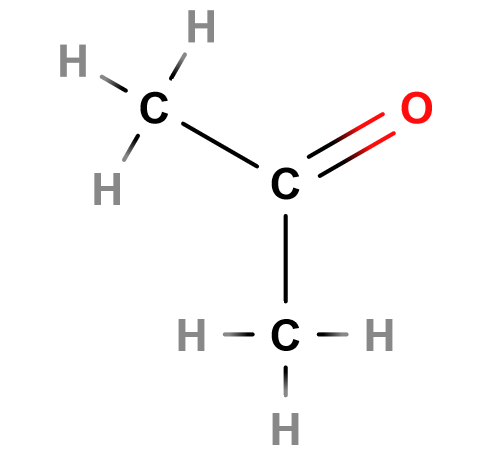
Carbonyls
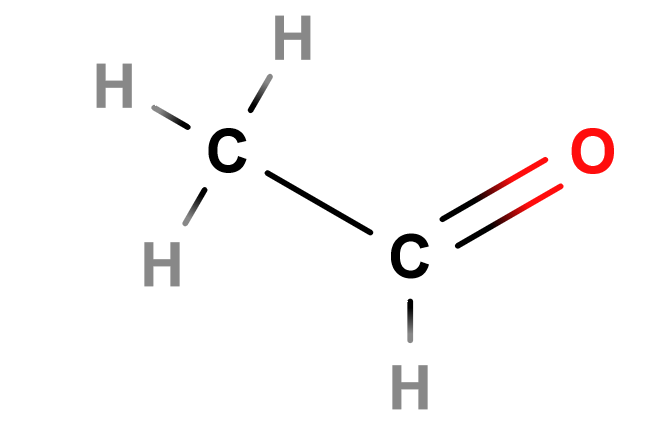
Lewis Structure of Ethanal
Notice the carbonyl group to the right.
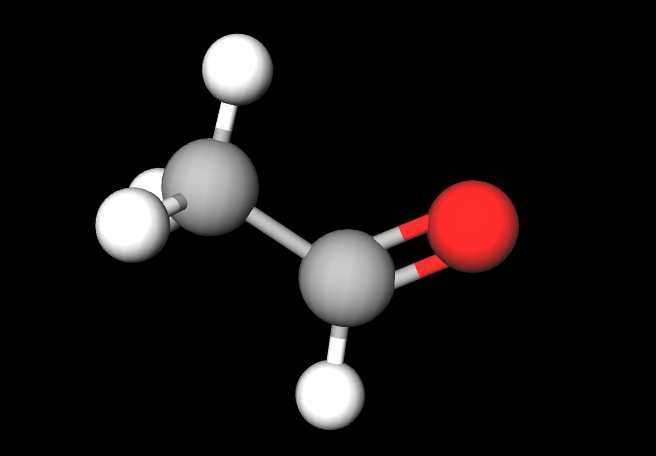
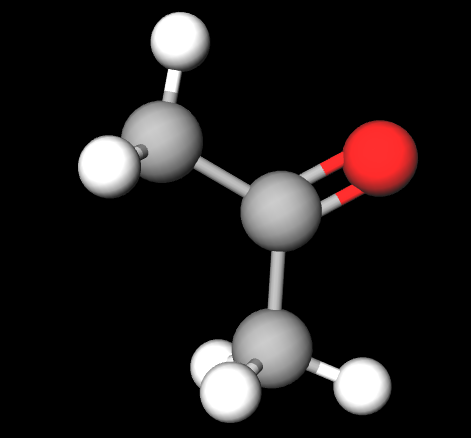
3D Ethanal
Carbonyls are a functional group that contains a carbon atom that is double bonded to an oxygen atom.
As such, carbon can form two more bonds - either a double bond with another atom or a single bond with two other atoms. Two classes of molecule come from the latter - aldehydes and ketones.
Aldehydes are carbonyl groups bonded to a hydrogen and an alkyl (general formula: R-CHO). Aldehydes have the suffix “-al”. Like alcohols, they take an alkane name as a prefix.
Ketones are carbonyl groups bonded to two carbon atoms. In other words, the double-bonded oxygen is attached to a normal hydrocarbon chain (general formula: RCOR’ - R’ just means it’s different than R). Ketones have the suffix “-one” (I hope you’re starting to see the pattern in these suffixes). They also take an alkane name as a prefix.
Lewis Structure of Propanone
3D Propanone Molecule
The above, officially, is called propanone, but maybe “acetone” is more familiar. Acetone is a chemical produced in the course of our normal metabolism. When fasting or when restricting carbohydrates (hydrocarbons with oxygen in them) in your diet (like on paleo or “keto” - notice the name), ketones, like acetone, are produced by the liver for energy.
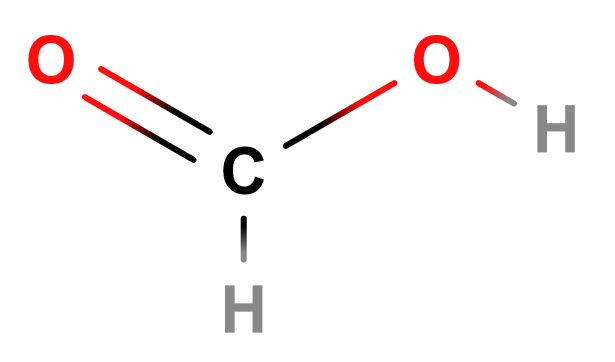
Carboxyl
Lewis Structure of Methanoic Acid
Above, I noted that carbonyls leave room for its carbon to form single bonds between two other atoms. What if carbonyl bonded with a hydroxyl group?
You would get carboxyls, a functional group which has a carbon bonded (double bond) to an oxygen and an “OH” group (general formula: -CO-OH).
Carboxyls form carboxylic acids (general formula: R-CO-OH) when they combine with alkyls. Carboxylic acids have the suffix “-oic acid”. Like most other functional groups, the prefix is the alkane name corresponding to the amount of carbons in the molecule.
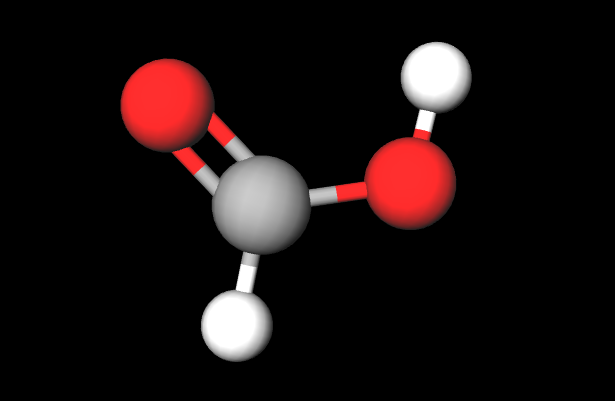
3D Methanoic Acid Molecule
Methanoic acid, or formic acid, is a part of sweat, but it’s best known as the defense mechanism that some ants use to drive away or even kill larger animals.
Carboalkoxy (Carbo-alk-oxee)
Carboalkoxy groups are similar to carboxyls, except the hydrogen in the hydroxyl group (-OH) is replaced by an alkyl (like CH3- or C2H5-). More specifically, the general formula of a carboalkoxy is -CO-OR’, where R’ is whatever alkyl.
This functional group creates the ester class of molecules (general formula: R-CO-OR’). The simplest ester has R’ as a methyl (CH3) group.
Naming esters is a little more complex than other functional groups, as it’s the first one we’re talking about that has a two-term name.
The first term is the name of the alkyl chain (or R in the formula).
The alkane name that corresponds to the number of carbons in the alkyl bonded to the oxygen (or, more simply, R’ in the formula).
The suffix “-oate”.
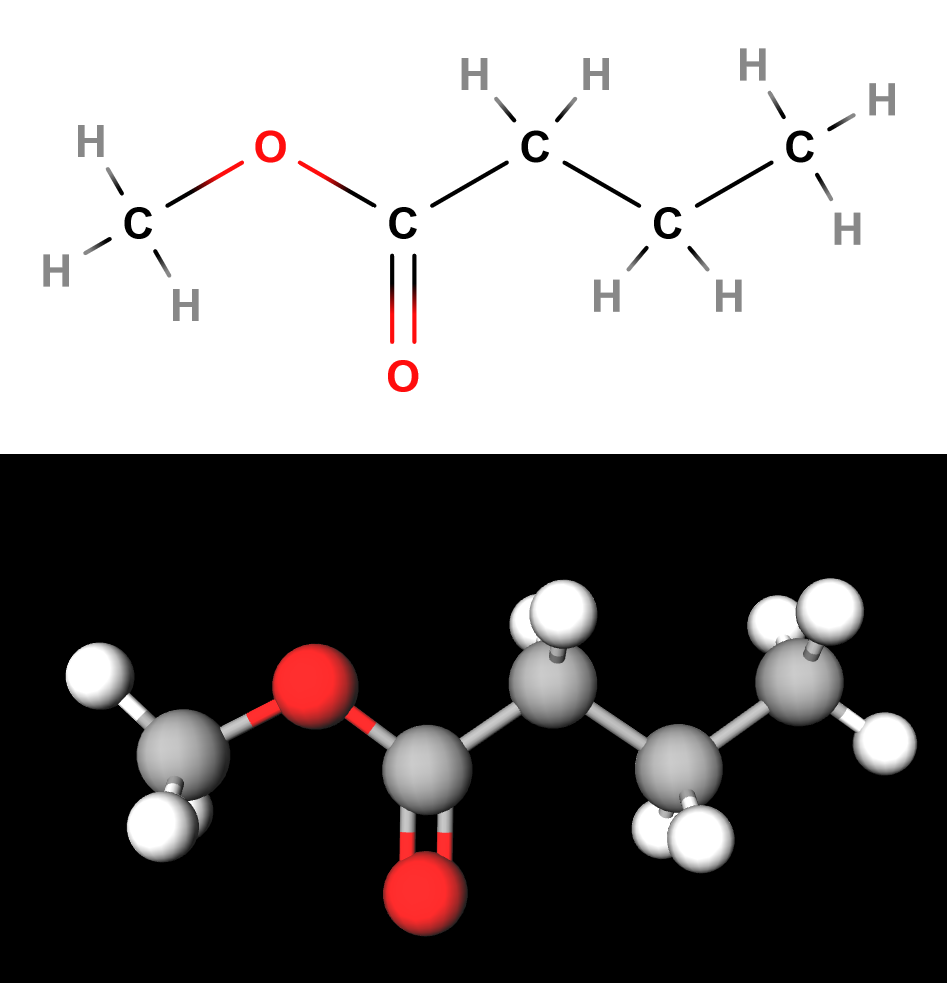
(Top) Lewis Structure of Methyl Butanoate; (Bottom) 3D Methyl Butanoate
Pineapple scents in perfumes may indicate the presence of ethyl butanoate.
Let’s walk through that name. First, notice that there is only one carbon after the oxygen in the carboalkoxy. We take the alkyl prefix that corresponds to one carbon - hence, methyl. Next, we look at the hydrocarbon chain on the other end of the carboalkoxy. In this case, there is four carbons. The alkane name of a four carbon molecule is “butane”. Lastly, we add the “-oate” suffix, giving us methyl butanoate.
This molecule, like most esters, has a fruity odor, and is even used in perfumes and artificial food flavorings.
Carboxamide
I bet that you could put together what this functional group is composed of, given its name. The general formula of a carboxamide is -CONR’R’’, where both R’ and R’’ is whatever alkyl or hydrogen atoms.
Carboxamides form amides. Funnily enough, as amines’ suffixes are their own name, amides have the suffix “-amide”. The prefix is the alkane name corresponding to the number of carbons.
Lewis Structure of Ethanamide
3D Ethanamide Molecule
Ethanamide is - personally - one of the most interesting molecules in nature. Astrophysicists were able to find this molecule at the center of our galaxy. This was an interesting find because amides such as these are responsible for forming peptide bonds, crucial bonds that form in the proteins of every living thing on Earth. In other words, astrophysicists found support for the theory that life can exist in space.
Summary of Functional Groups and Structures
Now I know that this seems like a lot. Students of organic chemistry are likely scoffing and crying, “That’s nothing!” But with all of that context out of the way, I can put everything above in a nice table for you guys to take a look at in the case you wanted to review.
| Functional Group | General Formula | Molecule Class | Suffix | General Formula | R |
|---|---|---|---|---|---|
| Alkyl | CnH2n+1- | Alkane | -ane | CnH2n+2 | - |
| Alkenyl | CnH2n-1- | Alkene | -ene | CnH2n | - |
| Alkynyl | CnH2n-3- | Alkyne | -yne | CnH2n-2 | - |
| Hydroxyl | -OH | Alcohol | -ol | R-OH | Alkyl |
| Amino | -NH2 | Amine | -amine | NR3 | Alkyl or Hydrogens |
| Carbonyl | R-CO-R' | Aldehyde | -al | R-CHO | Alkyl(R); Alkyl/Hydrogen(R') |
| Ketone | -one | RCOR' | Alkyl(R/R') | ||
| Carboxyl | -CO-OH | Carboxylic Acids | -oic acid | R-CO-OH | Alkyl |
| Carboalkoxy | -CO-OR' | Ester | Alkane + -oate | R-CO-OR' | Alkyl(R/R') |
| Carboxamide | -CONR'R'' | Amide | -amide | R-CONR'R'' | Alkyl(R); Alkyl/Hydrogen(R'/R'') |
With all of these established, there is only one more thing that goes into the naming of a molecule! But it’s also one of the most frightening. Some of you may know what I mean when I say that molecule names can be far, far longer than “methyl butanoate”. But, for the others, we’ll introduce those names in the next lesson as we walk through classifications of carbons.
For more thought-provoking content, join our mailing list!
See Flux’s Plans for 2020 here!