“Only words and conventions can isolate us from the entirely undefinable something which is everything.”
Science
Select the arc in which your journey will begin.
The Elemental Flow
The atom and it’s quantum origins
The Dance of Chemistry
What atoms do in the name of stability
Universe of Molecules
Molecules And The Rules That They follow



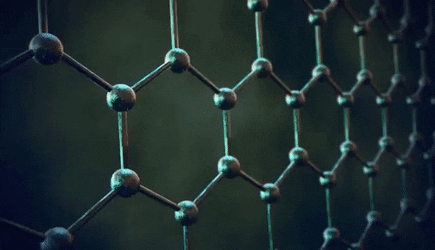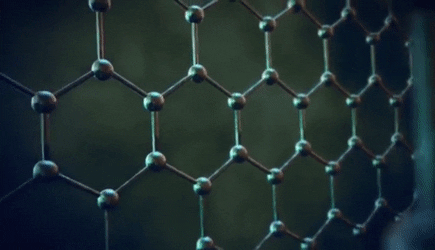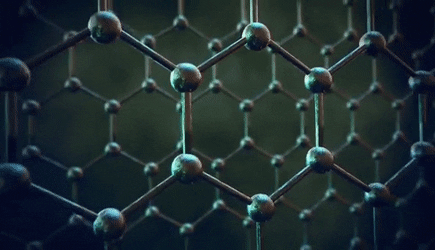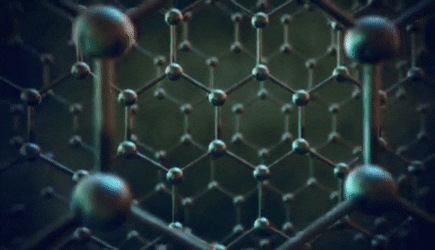






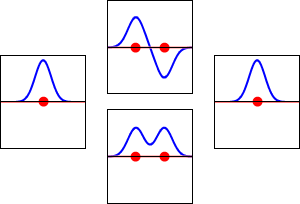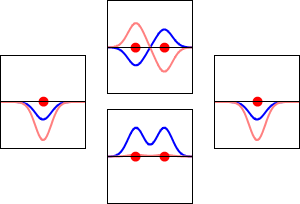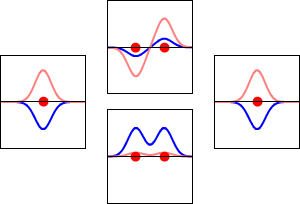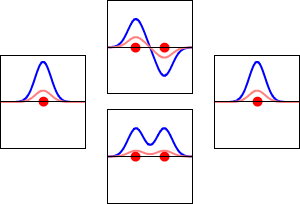


The significance of the Elemental Flow arc as it pertains to our reality.