7 - Advanced Theories of Covalent Bonding
Abstract (TL;DR)
The method for explaining bonding that we’ve gotten accustomed to only scratches the surface. When we factor in electron’s wave-like behavior, we see that bonding goes further than simply lone pairs and electrons between nuclei. A new bonding theory - molecular orbital theory - provides an explanation for how electron waves influence covalent bonding.
———
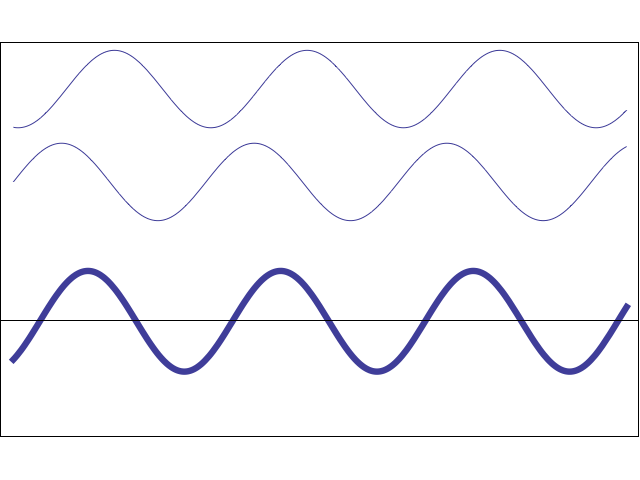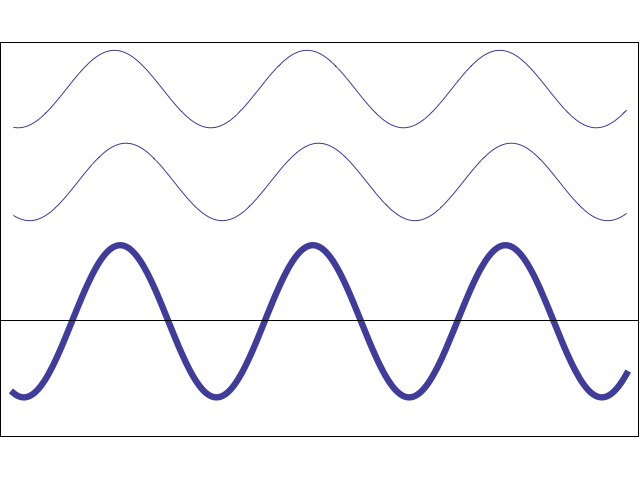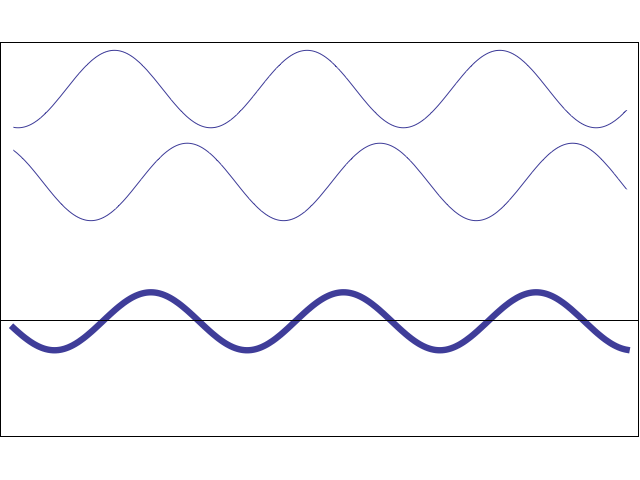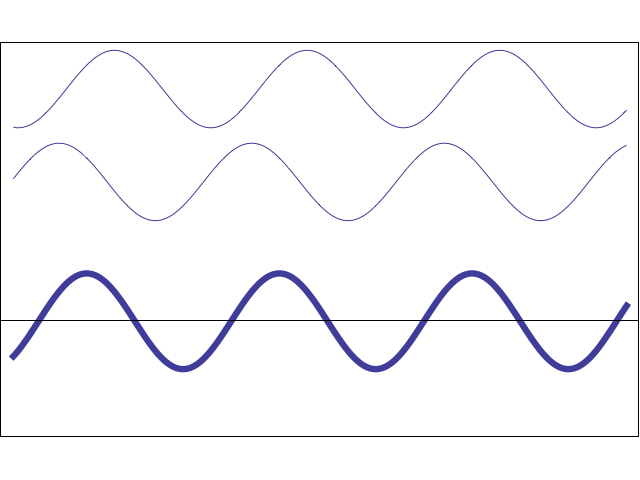
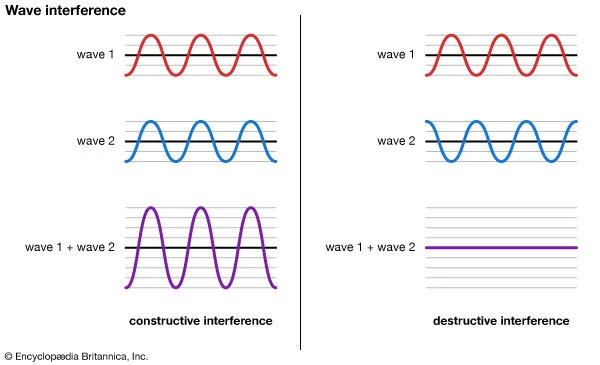
A waveform (middle) constructively and destructively interfering with a stationary waveform (top).
Image by Tamara Smyth, Department of Music, University of California, San Diego (UCSD)
So far, we’ve stuck to the idea of “lone pairs”, electrons being “between” nuclei, and hybridized atomic orbitals to explain a lot of different types of bonding. But this, somewhat, deviates from the ideas that we established when talking about the quantum mechanical properties of atoms.
Electrons are notoriously tricky particles; we know they exist because it wouldn’t make sense for them to not exist. Not only that, but we have been able to detect them through experiments since we learned that atoms have electrical properties in the late 1800s. However, even though we know they’re there, we have yet to see or even isolate them from an atom, relying on estimations like the electron cloud model or electron microscopy.
Valence Bond Theory
Knowing just how difficult it is to find where an electron is, how can we be so certain that electrons are only stuck in one position when bonded? Hybridized orbitals, partially, scratch that itch somewhat by defining sigma and pi bonds as “overlapping” orbitals that create a new kind of orbital - a new space for electrons to navigate.
As we’ve learned, these overlapping orbitals - and, by extension, atomic bonding - come about because of valence electrons - a representation of electrons that makes orbital theory and bonding easy to learn. What I didn’t reveal is that this was all a part of an overarching theory - the valence bond theory (VB Theory).
The valence bond theory encompasses everything that we have discussed to this point, covering that:
Electrons occupy orbitals that determine where they might be at any given point.
Electrons bond by attraction to another atom’s nucleus (or, more specifically, its effective charge).
Bonds are formed as orbitals overlap.
But there is a problem here - VB theory is limited in its scope. Firstly, and most importantly, it only accounts for the bonding activity of electrons.
For example, what if, at any point during a bond, the electrons move away from each other? Certainly, this is a possibility since electrons aren’t static particles.
Well, not only is this possible, it’s probable.
If we held that, in a bond, valence electrons from two atoms are near each other in between two attractive nuclei, even if given they had opposing spins, they’re still two particles with the same electrical charge - they will repel each other to reduce energy. What about the two nuclei themselves? If the electrons move far enough away from each other, the nuclei - both positively-charged - will repel each other too!
How does the VB theory account for this?
Molecular Orbital Theory
…Well, it doesn’t, at least not very well.
Though, fortunately, scientists recognized that there was more to bonding less than a century ago, in the middle of the quantum mechanics revolution, and developed molecular orbital theory.
Molecular orbital theory (MO theory) fills in the limitations by introducing a new way to envision electron activity and two new concepts.
To explain this covalent bonding theory, we must recall that electrons not only express particle-like behavior, but wave-like behavior. Waves, including wave-like particles, that come in contact with each other, as electrons do when they near each other, can interfere with each other.
This interference can take the form of constructive interference, where the energies of the two waves add to each other, creating a larger waveform, or destructive interference, where the energies of the two waves subtract from each other and create a smaller waveform.
In electrons, molecular orbital theory posits, both types of interference happen. When electrons engage in constructive interference, they strengthen each other, while those that engage in destructive interference weaken each other.
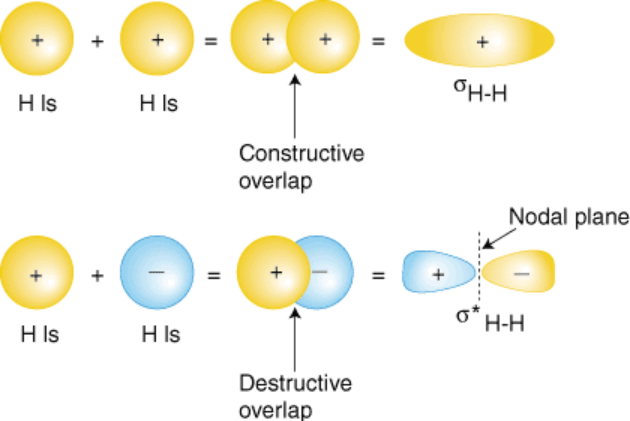
Bonding and Antibonding
Bonding tends to happen in areas where attraction between electrons and atomic nuclei is highest, while antibonding occurs where repulsion is highest.
You might imagine that constructive interference is good; it is. In fact, according to MO theory, this is why atomic bonding happens, as both electrons are “in phase” with each other. Destructive interference on the other hand is what happens when electrons repel each other - this is called antibonding, where electrons are “out of phase” with each other. Antibonding is exactly what it sounds like - a force that prevents bonding from occurring.
Here’s the kicker - the only kind of interaction covered in the valence bond theory is that of constructive interference. To discuss the effects of antibonding on a molecule, we would need to stay within the bounds of the molecular orbital theory. Hence: its importance.
Molecular Orbitals
Molecular orbital theory gives birth to the idea of a molecular orbitals, which, like atomic orbitals, provide the probable place of an electron, but over an entire molecule.
Some of you may wonder what differentiates molecular orbitals from hybrid orbitals, the type of orbital we discussed most recently. Hybrid orbitals form on an atomic level, as an atom reacts to another atom. Molecular orbitals, on the other hand, form at the molecular level, after bond has formed.
Sigma and Pi Bonds
Sigma bonds are covalent bonds brought about by head-on overlapping atomic orbitals. Pi bonds are covalent bonds from atomic orbitals that overlap side-to-side.
More important, perhaps, is that molecular orbitals are also a combination of two different orbitals - bonding and antibonding orbitals - each with different energy levels. This creates unique geometries as a result of attractive and repulsive forces within a molecule. Hybrid orbitals provide specific geometries to lower the energies of individual atoms.
Despite these differences, molecular orbitals and hybrid orbitals both involve the types of covalent bonding we discussed in the last section - sigma (σ) and pi (π) covalent bonds.
Molecular orbitals express covalent bonding somewhat differently than hybrid orbitals, however, due to the presence of the antibonding orbital. Both bonding and antibonding molecular orbitals involve sigma and pi bonding, meaning molecular orbitals are divided into four categories.
Gathering Orbitals
So, now, we have atomic orbitals, hybrid orbitals and molecular orbitals. For atomic orbitals, we utilized Hund’s Rules and the Aufbau Principle to organize electrons into its unique electron configurations. For hybrid orbitals, we utilized the phenomena of hybridization to explain how atomic orbitals change to reorder their electrons and become better suited for bonding.
Naturally, there also has to be a way to model how electrons are organized within these four categories of molecular orbitals. And that, my readers, will be the topic of the next lesson.
Read the CDC Guidelines on how to protect yourself during the current COVID-19 pandemic.
Vaccines are now available, with the Pfizer-based COVID-19 vaccine having full FDA approval for anyone ages 5 and above! Please check the CDC Website for instructions on how to receive one!
For more thought-provoking content, join our mailing list!
See Flux’s Plans for 2021 here!