8 - Drawing Molecular Orbital Diagrams
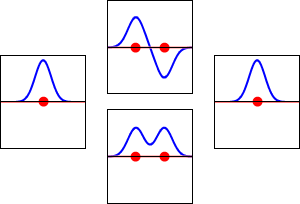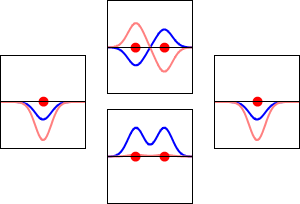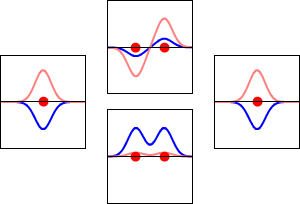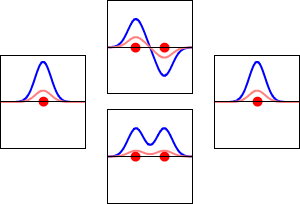
Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Although more complex, these diagrams reveal a more realistic case for bonding, allowing electrons to travel about a molecule, rather than in between one.
7 - Advanced Theories of Covalent Bonding
Valence bond theory, the theory that explains bonding through the use of lone pairs and electrons between nuclei, is convenient for explaining basic bonding. However, when we consider the wave-like behavior of electrons, it falls short in explaining the reality behind covalent bonding. This lesson moves us forward with a new theory - molecular orbital theory.
6 - Sigma and Pi Covalent Bonds
Hybridization, beyond giving us a reason for covalent bonding, also helps us understand that there are two different types of covalent bonds - the sigma and the pi bond.
5 - Bonding Orbitals and Hybridization
Hybridization, or the overlapping of orbitals to form new, hybrid orbitals, is a valuable missing piece to our understanding of covalent bonding.
1 - Elements and Chemical Bonds
Part 1 of the Dance of Chemistry Arc. Atoms, by all accounts, are not considered sentient, or living, yet they make up things that are considered living. In this arc, we will treat them as if they are a living civilization to learn about their behavior.