5 - Bonding Orbitals and Hybridization
Abstract (TL;DR)
Hybridization, or the overlapping of orbitals to form new, hybrid orbitals, is a valuable missing piece to the understanding of covalent bonding. These hybrid orbitals are what allow atoms to form the maximum amount of covalent bonds - something that our current, limited understanding of valence electrons doesn’t provide.
—
sp3 orbital
Image by Cdang via Wikimedia
Scientists established the exact angles and geometries between atoms through observation and experimentation. But, as always, where there is a will to explore something to its concrete conclusion, there is always an assumption that drove it. So, given our understanding that atoms bond to reduce their energy, that atoms use their valence electrons to do so and, when they do so, they take different shapes, the next course of action is to backtrack. What theory (or theories) explain this behavior?
Toward the end of the last section, I alluded to a relationship between orbitals and molecular geometry. And, it turns out that this relationship, like many things you will see from here on out, is based on science that we’ve already covered. Grab a seat: this one’s pretty cool.
Hybridization
Do you remember orbitals? Those things we learned about that were composed of those four quantum numbers? To recap: the principle quantum number (n) represents the electron “shell”, or the energy level of any given electron, the orbital quantum number (l) further divides those shells into smaller “subshells”, the magnetic quantum number (m) tells us the number of ways that subshells can orient themselves within a shell, and the spin quantum number (s) an electron’s momentum, or “spin”.
All four of these numbers come together to provide an understanding of paths that electrons can take within any atom, which take the form of specific shapes.
But what happens to these orbitals when atoms bond? Do they change shape? Do they disappear and form some completely different orbital?
No, it turns out that they remain as they are, but overlap with each other to create a unique hybrid of orbitals. We call this process hybridization.
Up until now, we’ve explained bonding using valence electrons. But hybridization gives us another way to explain covalent bonding - as a function of overlapping orbitals. However, since hybridization goes deeper than our valence electron-based framework, we can use it to explain: (1) the electron-pair geometry and (2) what a “covalent bond” really is.
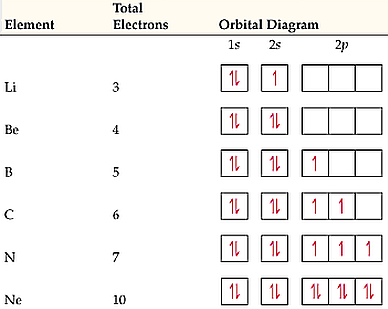
To conceptually understand what hybridization looks like, we have to revisit the orbital diagram - the familiar tool that we first used when discussing Hund’s Rules.
The Ground State
Before we get deep into how orbital diagrams help to explain hybridization, I’d like to go summarize the last two arcs in a few sentences (yes, really).
Atoms seek to lower their energy levels to be in their most stable state. To do this, the electrons within the atom occupy orbitals, specific areas in the atom, that keeps the energy low (since the energy in the atom rises the closer electrons are to each other). Atoms with electrons organized in their orbitals are in their ground state, or their most stable energy level.
Orbital diagrams show the way these electrons order themselves. In last column in the image above, we see numbers, letters, boxes and arrows. Each number (1 or 2, in this case) represents an electron shell (principle quantum number), the letter next to the number (s or p) represents subshells (orbital quantum number), each box represents an individual orbital and each arrow represents one of the two electron “spins” (spin quantum numbers).
Atoms that have complete orbital diagrams, where each orbital is filled with two opposing arrows, are known as noble gases (such Ne - neon - in the image above), the most stable atoms that exist in nature. But, those that don’t, can form bonds with other atoms, which brings the energy of the related atoms even further below their individual ground states, often making a molecule that is equally as stable as a noble gas.
Bonds occur when atoms with energetically negative valence electrons, the electrons on the outermost orbitals, “sense” the positive effective charge of another atom’s nucleus. This charge, brought about by the number of protons in the nucleus subtracted by the innermost “core” electrons, pulls the valence electrons between the two atoms, bonding them both. We call this bond “covalent” (co- meaning “joint”; -valent referencing “valence”).
The “Electron” Problem
With that summary, we’re ready for the new stuff. What you might not be ready for is the wrench, forcefully lobbed into our beautiful scientific machine.
Let’s take a look at a simple methane molecule and, more specifically, it’s central atom - carbon.
For any covalent bond to happen, each atom involved must “share” one of their valence electrons with another atom. In other words, carbon must have one electron in its outermost orbitals available. Carbon’s 1s and 2s orbitals are fully filled. The last orbital of its 2p subshell (the 2pz orbital) has no electrons in it, so it has no electrons to share from that subshell. But, in the other two p orbitals, carbon has one electron free, meaning it can form two covalent bonds.
But wait. Only two?
In methane, carbon is bonded to four atoms and, to become like a noble gas, it needs four electrons.
This is inconsistent with our understanding. How does any atom achieve a stability equal to that of noble gases if they have less electrons available for covalent bonding than they need to complete their outer shells?
Now, we have the key. Hybridization.
The Excited State
Right before a covalent bond happens, an atom’s energy increases, moving from their ground state to an excited state. When atoms become excited, its outermost orbitals combine to form new hybrid orbitals, better suited for covalent bonding.
*Hybridization Rule
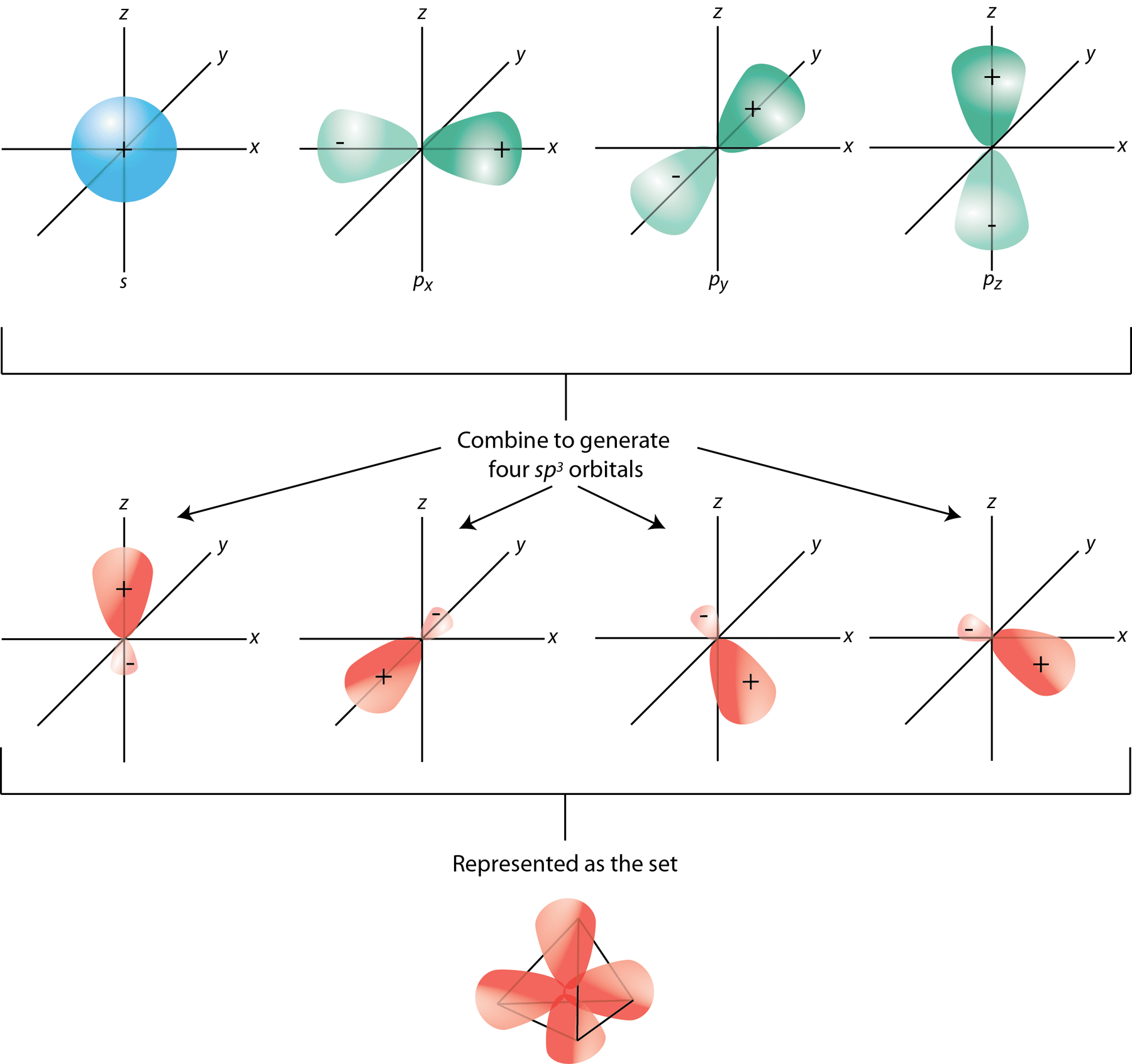
The 2s and 2p orbitals combine because they are closest to each other in energy. Only subshells that have similar energies can hybridize. The 1s and 2p orbitals, for example, can’t hybridize.
In carbon’s case, its outermost orbitals are in the 2s and 2p subshells. The one “s” orbital and three “p” orbitals combine and form four hybrid sp 3 orbitals that are equal in energy.* The energy equality is important - if the hybrid orbitals weren’t the same energy, that would impact their ability to form strong bonds.
The four electrons from carbon’s ground state 2s and 2p subshells are still there; they’re now a part of the new hybrid orbitals. However, we have to organize them properly, according to Hund's Second Rule. The rule explains that we fill each orbital with one electron spin before filling those subshells with its opposite spin. Therefore, the four sp3 orbitals each have one electron to work with.
With one electron in each of the four sp3 orbitals, there is an opportunity for four covalent bonds - exactly what carbon needs.
Now that we understand carbon’s hybridization, how about we bring in methane’s four hydrogen atoms?
*The sp and sp2 orbitals
Beyond the sp3 hybrid orbitals, which represents the tetrahedral electron-pair geometry, there are the sp2 and sp hybrid orbitals, which represent the trigonal planar and linear electron-pair geometries, respectively.
Each of four hydrogen atoms bonds with this hybridized carbon, overlapping their 1s orbitals with each of carbon’s four sp3 orbitals, creating the stable alkane. But there’s one more gift that hybridization gives us - the application of VSEPR theory. Why does each atom in methane organize themselves at exactly 109.5° apart? Because the sp3 orbital is shaped like a tetrahedral*. More specifically, each “lobe” of the hybrid sp3 orbitals are separated by 109.5° angles to avoid electron repulsion. Therefore, when the 1s orbitals overlap with these lobes, the entire molecule takes the shape of the sp3 orbital. (Beautiful.)
The Lone Pairs
Hybridization also explains why certain atoms have lone pairs. Take nitrogen, for example. Nitrogen has an electron configuration of [He]2s22p3, just one more electron than in carbon. Like carbon, it will also form sp3 hybrid orbitals (out of its 2s and 2p orbitals, since they are close in energy), except it will have another electron in one of its orbitals.
That extra electron, although seemingly insignificant, is important due to its position in the outermost orbitals. As we know, only electrons in the outer orbitals are used for bonding. Since we know that orbitals with one electron spin are used for covalent bonding, any orbitals with both their electrons are represented as lone pairs!
How Many Bonds?
Although we understand how covalent bonds happen, there’s still a bit more to go over on hybridization. For one, hybridization is not a static phenomenon; although I’ve shown carbon and nitrogen hybridizing, those are not the only ways those atoms (or any atoms) hybridize. This flexibility within the creation of hybrid orbitals not only explains how bonds form, but also how many, whether one, two or three, form. There’s still more about hybridization to discuss; stay tuned.
Be sure to sign up to FluxSci’s mailing list to see this post when it’s released in the coming week.
Read the CDC Guidelines on how to protect yourself during the current COVID-19 pandemic.
For more thought-provoking content, join our mailing list!
See Flux’s Plans for 2020 here!