6 - Sigma and Pi Covalent Bonds
Abstract (TL;DR)
Hybridization, beyond giving us a reason for covalent bonding, also helps us understand that there are two different types of covalent bonds - the sigma and the pi bond. Sigma bonds are those caused by a direct overlapping of atomic orbitals in between atomic nuclei while pi bonds are those caused by the overlapping of atomic orbitals away from atomic nuclei. We reveal in this section that sigma bonds exist within every covalent bond, while pi bonds only occur in double or triple bonds. Further, with an understanding of sigma and pi bonds, we can hybridize an atom at a glance.
——
Water Hybridization
Image via Kitasato University
Hybridization is wonderful for its capacity to explain bonding. It, however, is more flexible than just the few examples that I provided. In this part, we’re going to show just how flexible it is, starting with the two ways orbital overlapping can happen.
Sigma and Pi Bonds
We have explained that hybridization is caused by the overlapping of atomic orbitals to create new hybrid orbitals. These hybrid sp3 orbitals, as well as their sp2 and sp orbital cousins, have hidden more secrets for us - the sigma and pi bond.
These bonds, although sounding like something completely new are two types of covalent bonds. Sigma covalent bonds explain the head-on, lobe-to-lobe overlap of atomic orbitals between atoms’ nuclei.
But there is also an overlap that can happen between orbitals that are far away from the atomic nucleus. These bonds are pi covalent bonds.
These are the only two ways that covalent bonds form; without overlapping, covalent bonds can’t form, in which case, bonding would have to come as a result of ionic or metallic means.
Since the overlapping in sigma bonds is direct and linear, while that of pi bonds happens outside of the plane that the nuclei are on (above and below the nuclear plane), sigma bonds are a stronger covalent bond than pi bonds.
Sigma and Pi Bond Examples
We’ve dealt with methane in the last section, which provides a beautiful example of the creation of sigma bonds. In methane, the s orbitals of each hydrogen overlap with carbon’s hybridized sp3 orbitals linearly. Therefore, each bond in methane is a sigma bond. It has no pi bonds. To demonstrate pi bonds, then, we’ll have to navigate to a new molecule. How about ethene?
Like we did with methane, we’ll explain how the atoms in ethene hybridize, starting with the central atom…
Ah, right. This time, there is no central atom. So let’s use one side of the molecule.
In ethene, carbon covalently bonds to three atoms - two hydrogen atoms and one other carbon. How does this look in an orbital diagram?
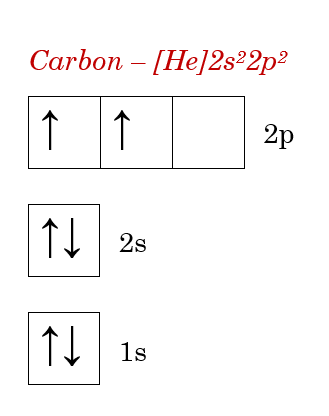
Carbon starts as it always does, at ground state. As atoms near carbon to bond, it moves to an excited state. But, unlike in methane, it only has to hybridize enough to meet its electron needs for three atoms.
This is what happens as the orbitals merge:
The carbon and two hydrogen atoms will bond with our carbon of interest in these new hybrid sp2 orbitals.
Now, for the elephants in the room. Why is that 2p orbital just hanging out there?
Well, since electrons, even those owned by other atoms, like to fill their lowest electron shells first, according to the Aufbau Principle. That means, since only two 2p subshells were needed to hybridize and the sp2 orbitals are lower energy than 2p orbitals (the 2s orbital involved in hybridization brings the energy of the hybridized orbitals down), only the sp2 orbitals were filled. The inevitable result was that 2p orbital being left out to dry.
Or is it?
Remember that there is no central atom here - the molecule is symmetrical; what happens to one carbon happens to the other. This means that the other carbon that has already formed a bond with our carbon of interest also has a 2p orbital free. I’ll give you one guess as to what happens between the 2p orbitals of both carbons.
If you guessed “they form a bond of their own”, you would be correct. Although this one is different, isn’t it? This bond wasn’t formed through hybridization, but more as a consequence of two atoms looking for an additional electron that the other one had. Now, I wonder where we discussed two different kinds of bonds before…
That, my readers, is the difference between a sigma and a pi bond. Each of the bonds formed during the hybridization process are direct, sigma bonds, where the last bond, formed simply by the nearness of the two atoms to each other, is a pi bond. Where each single covalent bond represents a sigma bond, the one double bond represents a sigma bond and a pi bond*.
Tricky Pi Bonds*
Don’t forget, even though there is an overlap both above and below an atom in this image, this doesn’t represent two different bonds! Orbitals are representations of the probable location of an electron in an atom. More concretely, in this image, an electron could be either below or above an atom in the p orbitals demonstrated in the picture. We represent the bond between p orbitals in a Lewis structure with a single line.
hybridization at A Glance
Knowing that single bonds are sigma bonds, double bonds are a sigma and a pi bond and, similarly, triple bonds are a sigma and two pi bonds helps us understand hybridization of an atom by just looking at the bonds.
Water. What is the hybridization of oxygen?
We know that sigma bonds represent a filled, hybridized orbital, as do lone pairs. Oxygen has two sigma bonds and two lone pairs, meaning there are eight electrons that must be accounted for in oxygen’s hybridized orbitals. Which hybridized orbitals contain eight electrons?
The sp3 orbital - a combination of one 2s orbital and three 2p orbitals (each containing spots for two electrons) - fits; oxygen is sp3 hybridized.
How about acetaldehyde?
The carbon on the left has four sigma covalent bonds, meaning eight electrons are involved in hybridized orbitals. That carbon is sp3 hybridized.
The carbon on the right has three sigma bonds and one pi bond. The pi bond is not involved in the hybridized orbital, similar to ethene above, but the three sigma bonds are, meaning six electrons are involved in the hybridized orbitals of carbon. That carbon is sp2 hybridized.
Oxygen. This Lewis structure is obscuring information, a charge I warned you guys about in the VSEPR Theory section . Remember, ask yourself how many valence electrons (or what the electron configuration) of an atom is before you analyze. Oxygen has six valence electrons - meaning there are two lone pairs not shown here. What is its hybridization?
Well…it doesn’t hybridize. Why?
Remember that hybridization only occurs to open up room for covalent bonds to happen. Hybridization will not happen if the atom already has enough room. In this case, oxygen, with its electron configuration of [He] 2s22p4, only needs two electrons to fill its valence shell and has two spots free. It doesn’t hybridize because it has no need to.
Dueling Theories
If hybridization has proved anything, it’s that most bonding can be explained using the methods we’ve discussed in the last two arcs. But these all assume that electrons are individual units that are stuck in between atoms.
There’s an issue with this ideology. When discussing the strides made by Louis de Broglie in the second section in the Elemental Flow arc (two years ago!), I mentioned that electrons are wily particles with two natures that made it difficult to know where an electron is at any given time. How do we, then, take the stance that electrons are immobilized in bonds?
In the next section, we introduce a different way of thinking about bonding that will challenge some of the ideas that we’ve established over the last year.
Science may be a river - but it certainly isn’t without its bumps.
Be sure to sign up to FluxSci’s mailing list to see that post when it drops. And make sure to follow the CDC guidelines on how to protect yourself during the pandemic.
Read the CDC Guidelines on how to protect yourself during the current COVID-19 pandemic.
For more thought-provoking content, join our mailing list!
See Flux’s Plans for 2020 here!