4 - Properties of Metals, Non-metals and Metalloids
Abstract (TL;DR):
This marks the end of the Dance of Chemistry arc. In this piece, we discuss properties held by the elements. Elements are divided between three characteristics: metals, non-metals and metalloids. Metals are malleable and are great conductors of heat and electricity. Non-metals are brittle and are bad conductors of heat and electricity. Metalloids are either brittle or malleable and can be good conductors of heat and electricity or not. Lastly, the characteristics of elements can determine the kinds of bonds they form.
—
Image via Tsinghua University Press and University of Science and Technology of China
So the atoms have fulfilled their lifelong dream of being cool enough to be among the noble gases by forming molecules. Here’s the problem: when you try so hard to fit in, it’s hard for your individuality to shine. However, no matter how hard you try, there will always be something about you that makes you different.
Let’s throw a spotlight on those differences and where they come from.
Properties of the Table
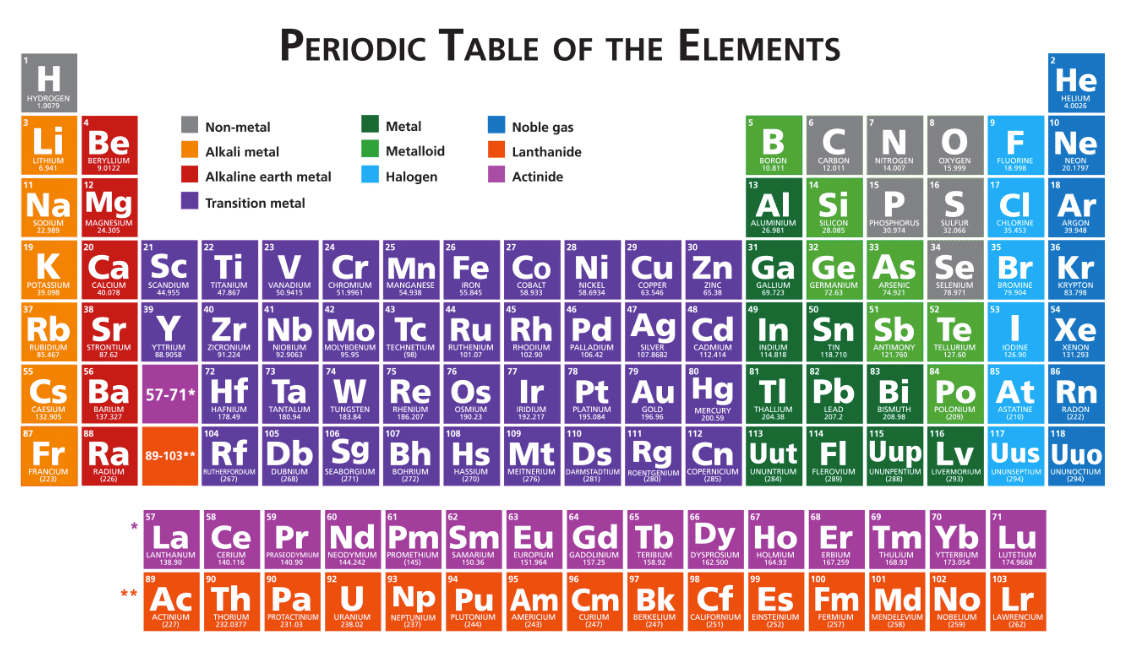
Did you notice that, on different periodic tables, there is, often, a large number of colors?
There are ten categories of elements, which are divided into three groups: metals, metalloids and nonmetals.
Aluminum (Metal)
Metals, which include alkali metals, alkaline earth metals and general metals, are:
Great conductors of electricity and heat
Malleable and ductile solids - meaning that they can be molded into sheets or wires without breaking or loosing their toughness
Shiny
Carbon (non-metal)
Nonmetals, including general nonmetals, halogens and noble gases, are:
Awful conductors of both electricity and heat
Typically brittle solids - meaning they break easily when being molded
Dull, but many colors
Silicon (metalloid)
Metalloids, as you may have figured, have properties that mimic both metals and nonmetals. They include general metalloids, transitional metals, lanthanides and actinides. Their properties include being:
Fair conductors of electricity and heat.
Because of this, they are called semiconductors, which are defined as materials that are neither good conductors or good insulators (opposite of conductor)
These are those same “semiconductors” that are used in computer parts!
Brittle solids
Shiny
A Program for the Dance
Those three groups are incredibly helpful for one major reason: in most cases, they offer you, my friend, insight as to what kind of bond will occur.
The bonding of two nonmetals forms covalent bonds. Carbon, one of the most important non-metals to us living beings, is famous for covalently bonding with everything. Electronegativity makes it so that each atom pulls on each other with similar strength, yielding a vigorous dance.
When two metals, which both are trying to give up their electrons, bond, they form metallic bonds. As these atoms are not very electronegative, they tend to be detached to their electrons, which the proton cores of other atoms sense and appreciate; even this waltz, gentle as it may be, creates a strong bond.
The bonding of metals and nonmetals, the two categories that inhabit opposite sides of the periodic table, often form ionic bonds. Nonmetals pull the metals to them, guiding them strictly, like a conductor that leads a orchestra’s rehearsal.
Metalloids are uniquely flexible partners. Whether bonding with other metalloids, or joining with metals or nonmetals, the dance of a metalloid is fully determined by the rules set by electronegativity. They are true chaos, forming either covalent, metallic or ionic bonds according to the properties of the atom that they choose to bond with.
Identity
The quest to be like something else comes with sacrifice. An atom’s identity is best displayed as it bonds, forming a unique relationship with its partner that can only be described as finding a soulmate - both atoms fulfilling each other’s need to be complete.
But unlike a true dance, which can end with a song’s last melody or at the will of the participants, atoms have no choice but to continue in this mutual relationship. The desire of all atoms to lower energy creates a common understanding that, once the total energy of all bonded atoms is lower, that is the configuration that they must stay in. In a way, atoms cease being metals, nonmetals or metalloids and just become one unified molecule; its very identity, including everything it was, changes.
The exception to this is if the substance is a pure substance, which is one that is made of only one kind of atom or molecule. A sheet of aluminum or tin, which is made of only aluminum or tin atoms, respectively, are pure substances. In the cases of pure substances, the properties of the entire material are the same as the properties one of the atoms in that substance, with the exception of strength and conductivity (both get stronger).
As sad as it may sound, there is beauty to be found everywhere within science. After all, without this dance of chemistry, life - existence - as we know it would not exist.
Conclusion
With this, the Dance of Chemistry arc comes to an end. The First Branch takes its place as the focus of our next adventure. With molecules making their appearance on the stage of science, we have a path forward to more complex molecules of chemistry and biology! But, until then, I feel as though we need more context on what we have done so far.
As always, feel free to ask questions if they arise and follow Flux’s community on Facebook and Twitter. Until next time…
Catch Flux's 2019 Agenda here. For more thought-provoking content, join our mailing list!