8 - Drawing Molecular Orbital Diagrams
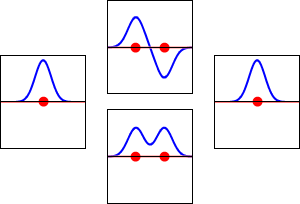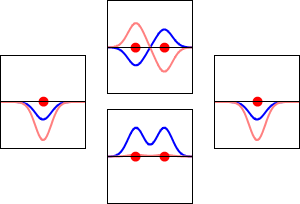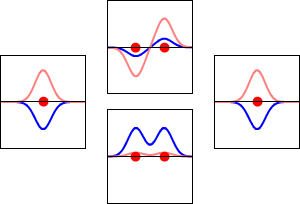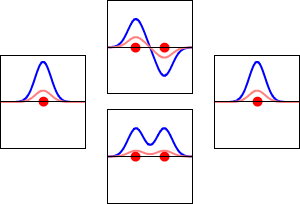
Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Although more complex, these diagrams reveal a more realistic case for bonding, allowing electrons to travel about a molecule, rather than in between one.
7 - Advanced Theories of Covalent Bonding
Valence bond theory, the theory that explains bonding through the use of lone pairs and electrons between nuclei, is convenient for explaining basic bonding. However, when we consider the wave-like behavior of electrons, it falls short in explaining the reality behind covalent bonding. This lesson moves us forward with a new theory - molecular orbital theory.
The Scientific Community's Great Weakness
The scientific community, as vast as it is, has a problem at its core. The culture of academia, the universities responsible for the lion-share of our modern-day research, is changing. And not in a good way.
2021 - Literacy, Bureaucracy and the Future of Flux
The 2021 Address of Flux Science, stating goals and aspirations over the coming year.
Hydroxychloroquine - A Case Study on Poor Science Communication
Pandemics are marked by the prevalence of a disease across a large population, including a global population. In fighting diseases, our greatest tool of offense is science and our greatest tool of defense is science communication. Yet the leadership of the United States has expressed open disagreement with our nation’s scientists and continued to push solutions that do not have the backing of science. What are the ramifications of this behavior on our society?
6 - Sigma and Pi Covalent Bonds
Hybridization, beyond giving us a reason for covalent bonding, also helps us understand that there are two different types of covalent bonds - the sigma and the pi bond.
5 - Bonding Orbitals and Hybridization
Hybridization, or the overlapping of orbitals to form new, hybrid orbitals, is a valuable missing piece to our understanding of covalent bonding.
4 - VSEPR Theory and Molecular Geometry
Did you know that molecules take different shapes based on the bonds that are formed? The VSEPR Theory helps us organize molecules into particular shapes according to how their electrons are distributed throughout the molecule.
3 - Classification of Carbon Molecules
Carbon forms the skeleton of all life on Earth due to its flexible nature. This part discusses the carbon’s flexibility through both its degree and priority and finishes our discussion on molecular naming.
2 - Functional Groups and Structures
Organic molecules are named using their functional groups, groups of bonded atoms that have distinct chemical properties. Here, we cover a few of those functional groups.