2 - Core Electrons and Valence Electrons
Abstract (TL;DR):
There is a distinction between electrons that are able to bond - valence electrons - and electrons that don’t - core electrons. Core electrons don’t bond because they, like noble gases, are stable, feeling the greatest amount of charge from the atomic nucleus. However, valence electrons feel an effective charge from the nucleus, or a charge brought about after the positive charge of the nucleus is subtracted by the number of core electrons. This effective charge is also felt by any valence electrons from other atoms, which is the main reason why stable bonds can occur.
—
Animation by Ahmed Saeed via Youtube
Before we continue talking about bonding, we have to talk about what urges atoms to do it. And since oxygen likes the attention, we’ll use it as a reference for this entire lesson. Good for you, oxygen.
As a reminder, oxygen’s orbital configuration is 1s22s22p4. This means that it has 8 electrons (add the exponents of the orbital configuration, which represent the spin quantum numbers). So, if it has an atomic number of 8, meaning that it has 8 protons and it also has 8 electrons, then why does it need to bond anymore? After all, it's negative and positive charges are balanced. It shouldn't be high in energy, right?
Not exactly.
Atomic Imbalance
Now, you are not wrong for thinking that 8 protons and 8 electrons should cancel each other out. 8 - 8 = 0. But that would only be the case if all 8 electrons felt the same force from the 8 protons and cancelled that force out. But that can't be, right? We know that as we increase in orbital level, the distance from the nucleus increases too. Therefore, the electrons closer to the nucleus feel more and more of its attractive force, while the ones farther away feel less; it's not a perfect cancellation. Also, remember the electron shield? This shield is made up of the innermost, or core, electrons, which "shield" not only the valence electrons from the nucleus' force, but also any electrons outside of the atom. Without this shield, bonding and chemistry as a whole would be veeeeery different. So let's talk more about these shielding core electrons.
The number of these core electrons is according to - you guessed it - energy. The lowest energy electrons are the least reactive, while the highest energy electrons are the most reactive. The highest energy electrons bond in order to lower their energy, the trait of valence electrons; the lowest energy electrons have no need to bond, the trait of core electrons. Hence, it’s not that there are, as our previous example states, just 8 electrons and 8 protons. There is a mixture of electrons that react and electrons that don’t in that 8, creating an imbalance of charge. We’ll come back to that later. First, how do you determine which electrons are core and which are valence?
It’s very simple. Remember that our noble gases are throwing a party for every atom and the only requirement is having the right amount of electron invitations. The standards to which an atom must meet are determined by the noble gases. So, let’s take another look at the periodic table.
Image via Schoolbag
Noble gases have full orbitals and are, thus, nonreactive, giving them the same traits as core electrons - low energy and a lack of a need to bond. Therefore, we can define all elements’ core electrons according to their previous noble gas. So, let’s revisit our 8 proton/8 electron, attention-seeking atom of the party oxygen. Its previous noble gas is helium - which is 1s2. That means that it only has two core electrons. Oxygen’s two electrons within its 2s orbital (2s2) and four electrons within its 2p orbital (2p4) are valence electrons. As such, oxygen can be written in core-valence orbital form as [He] 2s22p4.
This reveals that oxygen has eight protons, but only six, reactive valence electrons and, now that we know that oxygen actually has less reactive electrons than protons, which is true of any atom after helium, we can see why it reacts. Core electrons experience the full charge of the nucleus. But the valence electrons, shielded from the full charge by those core electrons, experience far less of a positive charge. Specifically, atoms after helium have nuclei that exhibit an effective charge on electrons outside of the core. This can be expressed in a simple mathematical form.
The effective charge is: Zeff = Z - S, where Z is the number of protons and S is the number of core electrons.
We know that oxygen has 2 core electrons, and 8 protons, therefore, it’s effective charge is 6 (8 - 2). The valence electrons feel this +6 charge (hence why there are 6 valence electrons). However, those valence electrons don’t contribute to the electron shield. And that means electrons outside of the atom can feel that charge too.
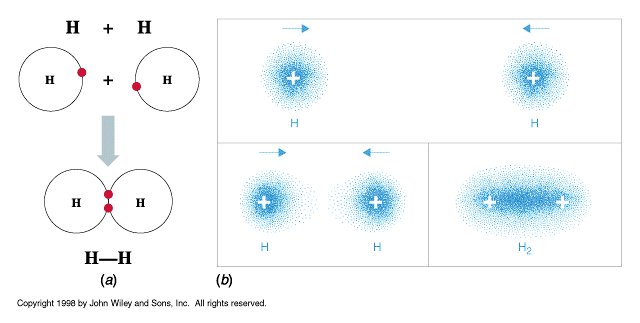
So how does oxygen use this? How does any atom? When the distance between compatible atoms is close enough, all valence electrons experience the effective nuclear charge from not only their own atom’s nucleus, but also the nuclei of the atoms that they are near. As such, they are pulled by the attractive forces of all relevant nuclei in the middle and their negative charge satisfies all relevant atoms. Sound familiar? You might recall something similar when we were discussing hydrogen in the last lesson. Yes, my curious friends - this is chemical bonding.
(a) The simplest expression of chemical bonding. (b) Notice how the cloud of blue is at its darkest in between the atoms or near the protons?
Image by John Wiley and Sons, Inc.
The Bonding Summary…
As the amount of protons increase, the effective nuclear charge must increase. But the core electrons don’t change in number until you get to the next row on the periodic table, after the previous noble gas goalpost has been met. For example, the entirety of the second period, from lithium (Li) to fluoride (F) has two core electrons because helium was their last noble gas and, therefore, sets the precedent for core electrons, due to stability. Those core electrons feel the full force of the nucleus, but they are often not enough to completely mask the full positive charge. Therefore, not only do valence electrons within an atom feel the nucleus’ pull, but so do the valence electrons outside of an atom that are close enough to it. Since an atom wants a complete valence shell, in order to be as stable as a noble gas, it freely attracts these valence electrons. But the valence electron of another atom also feels a pull due to the effective charge of its own nucleus. Therefore, the two atoms must share their electrons. The result is that both atoms improve their stability, potentially even having enough electrons to be as stable as a noble gas and, therefore, being able to party with their idolized noble gases.
Now I can tell you what makes oxygen so bad…in the next part. But, I wanted to leave you with a question.
What makes hydrogen and helium so different in terms of core and valence electrons?